Malaysian sufferers of a deadly muscle-wasting disease are denied the latest drugs

There is still no cure for a fatal but rare muscle wasting disease named after a 19th century French neurologist that affects about 300,000 boys worldwide. About 3,000 Duchenne muscular dystrophy patients are in Malaysia and as many as 300 of them are in Sabah. But new drugs developed in the last six years that hold promise of a cure have been denied them because of their prohibitive costs. Ironically, it is a Sabah woman, whose son suffers from Duchenne, who has contributed significantly to the development of the first three exon skipping drugs ever to have been approved by the American Food and Drug Administration to treat the deadly disease. More recently, the FDA has approved the first gene therapy which doctors say may be close to a cure for Duchenne. But none of the Malaysian patients, particularly those in Sabah, can avail themselves to these. A year’s treatment with Amondys 45 (casimersen), an exon skipping drug, can cost as much as RM6.5m ($1.5m) while a one-time gene therapy with Elevidys (Delandistrogene moxeparvovec) costs RM14.2m, making it one of the most expensive drugs in the world.
An exon is a segment of a gene. Skipping over faulty exons helps the dystrophin gene to produce more protein, the lack of which causes muscular degeneration. Elevidys is a micro-dystrophin that is injected into muscle cells to replace the inherited mutated gene.
Catherine Jayasuriya, 63, whose Coalition Duchenne has been raising funds for Duchenne researchers for 13 years, wants these drugs to be available in Malaysia but doesn’t say how. She said she was working at it when she was in Kota Kinabalu in July to launch her 12th annual Mount Kinabalu expedition to raise global awareness of the Duchenne scrouge. She is the eldest of two children and an only daughter of the late Thomas Jayasuriya, a prominent Sabah lawyer and former High Commissioner to Canada. She still considers Sabah her home despite having been living in California with her civil engineer husband, two sons and a daughter.
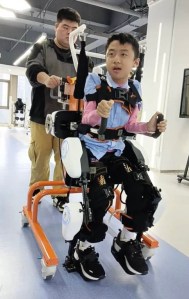
Sabah Duchenne patients are treated at the Likas Women’s and Children’s Hospital, a public hospital, with free corticosteroids such as prednisone that could slow down the weakening of muscles. These drugs cost between RM5 and RM50 per prescription at a private pharmacy. Ambu bags (manual respirators) and other breathing devices donated by Coalition Duchenne are given to patients who need them to breath. The Coalition has been donating RM20,000 a year for the last three years to the hospital to pay for equipment for Duchenne patients which the hospital does not provide. Wearable robotic devices known as exoskeleton to aid mobility are costly. The one worn by 12-year-old Rayce Low Rok Chun (picture) looks bulky and cumbersome. It is out of reach of most patients as it costs a hefty RM150,800.
Research into Duchenne had been painstakingly slow. It was not until the 1980s when medical researchers found that Duchenne is inherited from a mother’s flawed gene that the race to find its cure has intensified. Dr Louis Kunkel, an American geneticist of the Boston Children’s Hospital, and his team identified a mutated dystrophin gene on the X chromosome that causes Duchenne muscular dystrophy which affects mostly boys (one out of 3,300) who inherit their mothers’ defective X chromosome gene. Boys have an X and a Y chromosome which determines their sex. Girls seldom develop the disease (one out of 50m) because they have two X chromosomes of which the good gene will compensate for the bad one. It is very rare for a girl to have two copies of a flawed X chromosome gene, according to doctors.
Yet Jayasuriya says that information on and awareness of Duchenne were lacking when her eldest son Dusty was diagnosed with Duchenne in 1997 when he was five. Dusty is 31. His younger brother Lucas, 29, doesn’t have Duchenne. Most Duchenne patients live to their early or mid-20s and die from breathing difficulties and heart failure as their diaphragm and heart muscles weaken. Jayasuriya founded Coalition Duchenne in 2011 and the next year launched the Mount Kinabalu Expedition by inviting mountain climbers from different countries to scale Malaysia’s tallest peak at 4,095m (13,435ft) to raise awareness of Duchenne. More than 700 people from as far as Europe and Africa have joined the expedition. The mountain hosts the world’s toughest mountain race, the Mount Kinabalu Climbathon, which is returning this year on October 6 after a six-year absence.
Now 75 drug companies from all over the world including Singapore and Japan have joined the race to find a definitive cure for Duchenne. In fact NS Pharma, a subsidiary of Japan’s Nippon Shinyaku company, develops the fourth FDA-approved exon skipping drug Viltepso (viltolarsen). The other three: Exondys 51 (eteplirsen), Vyondys 53 (golodirsen) and Amondys 45 (casimersen) are made by Sarepta Therapeutics, an American drug company, which are based on the work of Dr Steve Wilton of the University of Western Australia which Coalition Duchenne funded.
More recently Jayasuriya says her Coalition has found that the scarring of a Duchenne heart is similar to that from a heart attack. “We convinced Dr Eduardo Marban, (of the Cedars Sinai Medical Center in Los Angeles) to apply his stem cell therapy to that scarring. That has now become a treatment for Duchenne that will potentially be approved by the FDA next year,” she says. Dr Marban says Jayasuriya is the first advocate of his stem cell therapy known as Dramiocel, and gave his Cedars Sinai its first seed fund for his research. Dramiocel has shown to slow down Duchenne progression significantly and to improve heart function.
Since its founding, Coalition Duchenne has raised more than $3m to fund Duchenne research. The sum may sound modest when compared to the more than $200m spent by the Muscular Dystrophy Association. But it has produced results. And Jayasuriya remains sanguine. “Years and years have passed. We have been waiting for treatments,” she says. “And now we are on the cusp of significant progress. Hope is in sight, with the FDA now approving eight new therapies, and many more to come.”